Our group is based in the Photon Science Institute and the School of Chemistry, University of Manchester, UK
Chemical reactions on surfaces are the driving force behind many important technologies, most notably heterogeneous catalysts and electrochemical technologies such as batteries and fuel cells.
The group uses cutting-edge surface characterisation techniques to understand surface chemistry on the atomic scale. We have a particular interest in studying surface chemistry in-situ (that is, following surface chemical reactions as they happen).
Research Areas
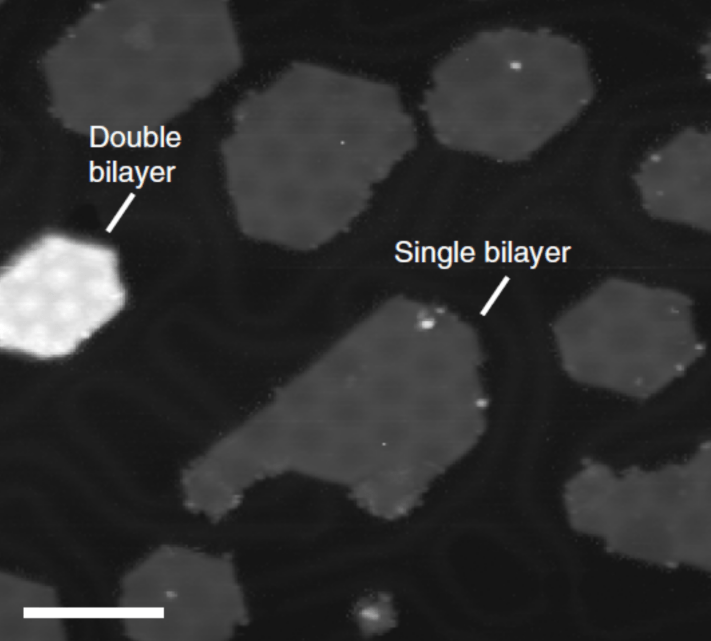
2D materials as electrocatalysts
Novel electrocatalysts are needed for future electrochemical technologies in the energy generation and storage sector. For example, large amounts of platinum is needed to catalyse the Oxygen Reduction Reaction (ORR) in a hydrogen fuel cell. An efficient, earth abundant and poison-resistant alternative is highly desirable. Nitrogen-doped graphene (graphene with some of the carbon atoms replaced by nitrogen) is a promising candidate for an earth-abundant alternative, but development is hampered by a poor understanding of the active sites for the catalysis and the reaction mechanism.

Similarly, a new class of 2D materials, the layered oxides, are promising candidates for the Oxygen Evolution Reaction (OER) – half of the electrochemical water splitting reaction needed to produce hydrogen.
We use a combination of surface-sensitivity spectroscopy (X-Ray Photoelectron Spectroscopy) and atom-resolved microscopy (Scanning Tunnelling Microscopy/Atomic Force Microscopy) to understand the surface composition and chemistry of these materials on the atomic scale. We can then relate the surface structure and chemistry to their catalytic activity and gain real insight into their catalytic mechanism.
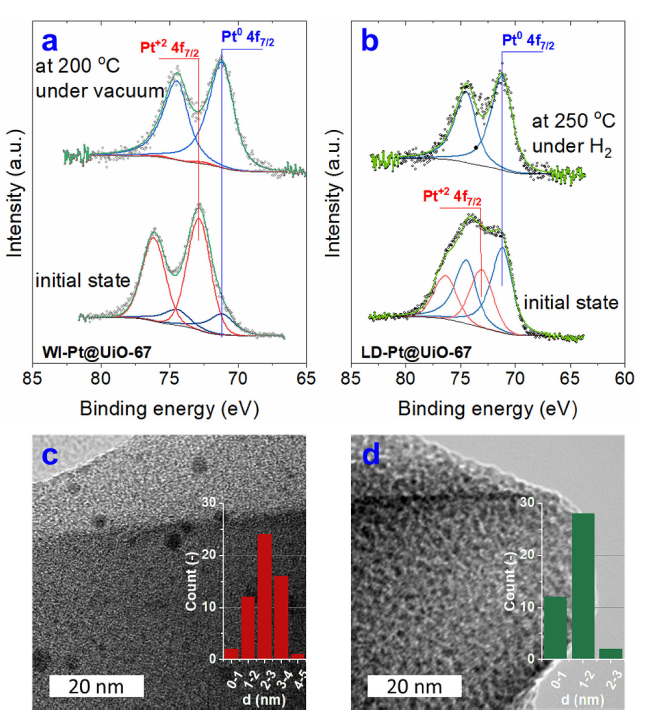
Operando studies of heterogeneous catalysts
Heterogeneous catalysis is enormously important in the chemical industry. It’s estimated that 95% of all products (by volume) have been through a catalytic process at some point during their manufacture. Despite this, understanding of heterogeneous remains poor due to the difficulty of characterising the surface of the catalyst during operation. Post-mortem studies of catalysts are of limited use as the surface of the catalyst may be (and often is) completely different under operating conditions.
A new generation of in-situ characterisation techniques are now emerging which allow researchers to perform operando studies of catalysts (that is, characterise them whilst they are converting reactants into products). Our group uses Near-Ambient Pressure XPS to look at the operando surface chemical composition of catalytic material and gain new insight into their reaction mechanism.
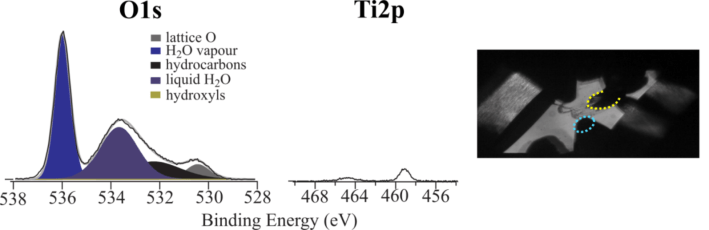
Probing the solid-liquid interface in-situ
Electrochemical processes take place at the electrode-electrolyte interface. To fully understand electrocatalysts and battery electrodes, it is essential to characterise this interface during electrochemical reactions. However, as the interface is buried by the solid electrode on one side and the electrolyte on the other, it is a major technical challenge to study this critical interface. Our group are currently developing novel and cutting edge methodologies using our Near-Ambient Pressure XPS system to study the electrode-electrolyte interface in-situ – based on establishing an ultrathin wetting layer over the analysis area, through which the interface can be measured.